Chances are pretty good that at some time in your life, you’ve crossed paths with a norovirus. And chances are that you remember the encounter vividly, or at least its aftermath. I recall a run-in with the bug one Christmas, when my parents brought over more than just toys for the kids when they visited. Within a day, everyone in the house was sharing the joy. Twas the season; they don’t call it the winter vomiting bug for nothing.
Most of the 685 million norovirus infections each year resolve after a few miserable days, but some require hospitalization and 200,000 of them result in death, mainly from dehydration and mainly children. An easy to use, cheap, and accurate means of detecting the virus in the field would be quite a boon to public health. And soon, smartphones may be able to do just that.
FROM LAB TO FIELD
There’s plenty of debate about whether viruses constitute life or not, but one thing is for certain: viruses are perfectly adapted to their mission. Consisting of little more than a stripped-down genome, viruses represent the minimal feature set needed to replicate in a host cell. As such, there’s not a lot to go on when trying to identify viruses; they have no metabolism of their own, they don’t grow by cell division, and they’re far too small to be seen with anything short of an electron microscope.
In the lab, viruses are usually identified by methods that target either the genome or the protein coat that protects it. Genomic methods include polymerase chain reaction (PCR), which amplifies the tiny length of DNA or RNA within the virus (amplifying the genome of RNA viruses like noroviruses requires first turning the RNA into DNA with an enzyme called reverse transcriptase; that process is known as RT-PCR.) PCR methods produce billions of copies of the genome which can then be easily detected using gel electrophoresis or other methods. Detecting the protein capsule is done with immunoassays, where antibodies specific for one of the coat proteins bind to the virus particles; those antibodies are then detected by anti-antibodies that have either a radioactive or fluorescent tracer attached to them.
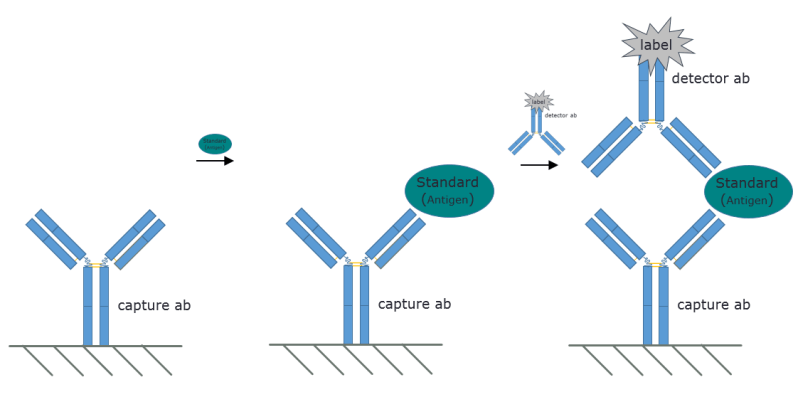
While both of these methods are simple in the lab, they are impractical in the field and require expensive instrumentation. To solve the problem of rapidly identifying norovirus in real-world conditions, a team at the University of Arizona has developed a unique microfluidics virus detection method. The idea is similar to the “sandwich” immunoassay used in the lab, but with a few twists.
FLUORESCENT CLUMPS
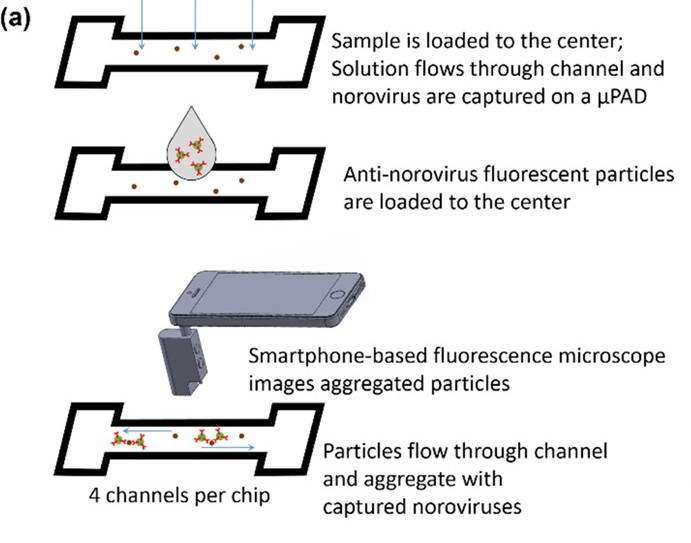
The first innovation is the use of microfluidic paper. The paper is a small piece of nitrocellulose filter paper, also known as flash paper, upon which small channels have been printed with a solid-ink printer. The paper is then heated to melt the waxy solid ink into the pores of the paper, forming a hydrophobic barrier. A small amount of aqueous sample suspected to be contaminated with norovirus is added to the paper; the sample absorbs into the pores of the paper through capillary action and spreads out along the channel, but is restrained from migrating very far by the waxy barriers.
Next, a fluorescently tagged antibody specific for one of the norovirus coat proteins is added to the channel. The antibody solution is rapidly pulled into the paper, where it binds to any norovirus particles. The antibodies pile onto each particle, forming large fluorescent clumps that are easily detected under a microscope. Normally this is done with a fluorescence microscope in the lab, but the team has a trick for that too.
Using an off-the-shelf optical microscope and a bandpass filter for 480 nm, they turned an ordinary smartphone into a portable, purpose-specific fluorescence microscope. The microscope clips over the camera lens while the filter goes over the white LED to provide the blue excitation light needed to visualize the clumped fluorescent antibody conjugates; the team points out that a separate blue LED would work too. Thanks to the microfluidics, the clumps are well distributed along the channel, making them easier to count with image analysis software that runs right on the phone. The more clumps, the higher the viral load in the sample.
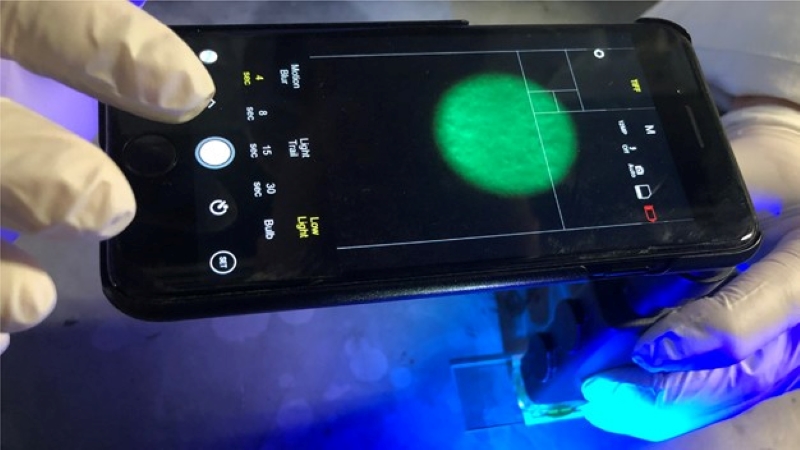
The image analysis on the phone is based on MATLAB. The fluorescent antibodies emit light at around 525 nm when stimulated with blue light. The program uses the data from both the red and green channels of the camera while ignoring the blue, capturing the emitted wavelength while blocking the excitation light. Fluorescent bodies are isolated from the background noise and counted if they exceed a cutoff size. The app generates a reading in as little as a minute, with no need for an Internet connection.
The whole hardware setup costs about $50, not including the phone. The team was able to show that the system can detect norovirus down to a single copy of virus per microliter of pure water, or 10 copies per microliter in reclaimed wastewater, a more realistic field scenario. The whole kit is easily and cheaply assembled, no particular expertise is needed to operate it, and all the materials and reagents, including the antibodies and the fluorescent tags, are commercially available.
Infectious agent detection innovations like this could really be a boon to public health. Leveraging the ever-growing computing power and sensor capabilities of smartphones might just lead to detection modalities for a wider range of pathogens with increased sensitivity, which could lead to better prevention and increased quality of life for millions.
[via Tech Xplore]
No comments:
Post a Comment