In my five-part series on coronavirus and the failure of big government (here, here, here, here, and here), the Food and Drug Administration (FDA) received some unflattering attention.
Whether we’re examining its performance regarding equipment, testing, or vaccines, the bureaucracy has hindered the private sector’s ability to quickly and effectively respond to the pandemic.
 Today, let’s devote an entire column to problems with the FDA.
Today, let’s devote an entire column to problems with the FDA.
Historically, the big issue is that the bureaucracy is too cautious and risk-averse.
The argument from the FDA is that a lengthy and expensive process for approving drugs is necessary to avoid the risk of a drug with bad side effects.
And there are benefits to that approach, with thalidomide being the obvious example.
However, there are also costs. Most notably, the FDA’s onerous approval process means that it takes a long time before consumers get access to many life-saving and life-improving drugs.
The net result is that the FDA has killed more people than it has saved.
If you think that is hyperbole, read this summary of academic research from the Independent Institute.
…requiring a lot of testing has at least two negative effects. First, it delays the arrival of superior drugs. During the delay, some people who would have lived end up dying. Second, additional testing requirements raise the costs of bringing a new drug to market; hence, many drugs that would have been developed are not, and all the people who would have been helped,
even saved, are not. …three bodies of evidence suggest that the FDA kills and harms, on net. …It is difficult to estimate how many lives the post-1962 FDA controls have cost, but the number is likely to be substantial; Gieringer (1985) estimates the loss of life from delay alone to be in the hundreds of thousands (not to mention millions of patients who endured unnecessary morbidity). …Deaths owing to drug lag have been numbered in the hundreds of thousands. …in recent years thousands of patients have died because the FDA has delayed the arrival of new drugs and devices
Oh, and it’s worth mentioning that the FDA process means companies much charge higher prices to compensate for the expensive approval process.
But let’s look at where we are today and explore the FDA’s role in fighting the coronavirus.
We’ll start with this tweet about the bureaucracy’s unhelpful role last year as the pandemic was getting worse.
But I mostly want to focus on what the FDA is doing today to make our lives less safe.
Professor Garret Jones of George Mason University has a column in the Washington Examiner excoriating the bureaucracy’s deadly delays in approving another vaccine.
Good enough for Britain. Good enough for the European Union. Not good enough for the United States. That’s what the U.S. Food and Drug Administration thinks about the evidence for the Oxford-developed, AstraZeneca-made COVID-19 vaccine: the cheap, refrigerator-friendly, easy-to-transport injection that, so far at least, is 100% successful at keeping people with COVID-19 out of the hospital.
The Oxford vaccine has been given to more than a million British citizens, and the EU is now scrambling to find as many doses as it can… So why hasn’t the Oxford vaccine been approved for use in the U.S.? Because the FDA made clear that AstraZeneca needed to finish its lengthy trials in the U.S., above and beyond the trials AstraZeneca had already run in the United Kingdom, Brazil, and South Africa. …My colleague at George Mason University, Alex Tabarrok, refers to the “invisible graveyard” — those dead because lifesaving drugs and vaccines were delayed or never invented. Every day we delayed vaccine approval in 2020 was a day that COVID-19 could spread unabated, killing people in the U.S. in the hundreds of thousands. And that deadly delay continues in 2021. …The FDA should approve the Oxford vaccine immediately. Since it doesn’t require fancy freezers, it will easily reach small towns and local clinics in a way that current COVID-19 vaccines in the U.S. can’t.
Since I have friends who have died from the virus, it’s infuriating that the FDA is hindering the approval and deployment of the AstraZeneca vaccine.
Heck, I would love the chance to get it myself, yet a bunch of cossetted bureaucrats are telling me that my life should be at risk instead.
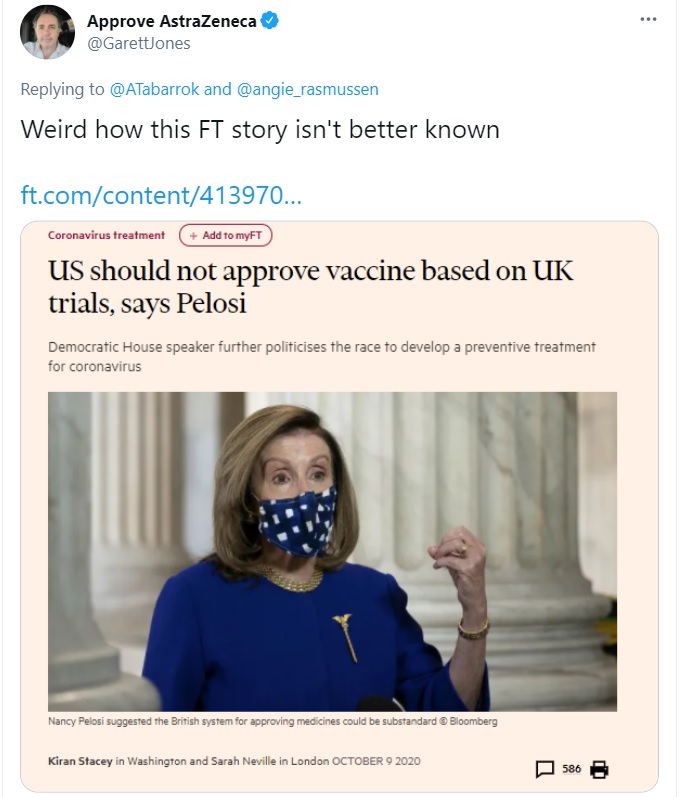
If you’re wondering why the FDA is mindlessly causing needless danger and death, this tweet from Professor Jones may tell us everything we need to know (he also mentioned Pelosi’s unhelpful role in the column cited above).
Why is she putting people’s lives at risk?
Is it because she reflexively supports red tape? Is it because she’s getting campaign contributions from Pfizer and is trying to keep a competing vaccine off the market? Is it because Astra-Zeneca’s vaccine was developed in the U.K. and she opposed Brexit?
I don’t know the answer, but I’m 99.99 percent sure she’s already been vaccinated and isn’t at risk like the rest of us.
What about the FDA’s motivations?
Dr. Henry Miller’s recent column in the Wall Street Journal has some insight on why the bureaucracy is willing to put our lives in danger.
…countless patients could benefit, if Food and Drug Administration regulators were less risk-averse. I know that from firsthand experience. …As the head of the FDA’s evaluation team, I had a front-row seat. …during the early 1970s, as the supply of animal pancreases declined and the prevalence of diabetes increased, fears of drug shortages spread. Around the same time, a new and powerful tool—recombinant DNA technology, or gene splicing—became available. …Eli Lilly & Co. immediately saw the technology’s promise for producing human insulin…
Insulins had long been Lilly’s flagship products, and the company’s expertise was evident in the purification, laboratory testing and clinical trials of Humulin, its new human insulin. Lilly’s scientists painstakingly verified that their product was pure and identical to pancreatic human insulin. …In May 1982 the company submitted to the FDA a voluminous dossier providing evidence of the product’s safety and efficacy. …My team and I were ready to recommend approval after four months’ review. But when I took the packet to my supervisor, he said, “Four months? No way! If anything goes wrong with this product down the road, people will say we rushed it, and we’ll be toast.” That’s the bureaucratic mind-set. …A large part of regulators’ self-interest lies in staying out of trouble. One way to do that, my supervisor understood, is not to approve in record time products that might experience unanticipated problems.
Sadly, this FDA mindset hasn’t changed.
As a result, Americans are needlessly dying.
P.S. Professor Alex Tabarrok has another example of senseless regulation from the FDA.
P.P.S. Here’s my column on the CDC’s unhelpful role in dealing with the pandemic.
P.P.P.S. And here’s what I wrote about the international bureaucrats at the World Health Organization.
P.P.P.P.S. When dealing with other advanced nations, we should adopt the principle of “mutual recognition” so our consumers have the option of benefiting from products approved elsewhere, such as the Astra-Zeneca vaccine.
P.P.P.P.P.S. In an all-too-typical example of Mitchell’s Law, politicians and bureaucrats have created a process than makes drugs very expensive. They then respond by agitating for price controls rather than fixing the underlying problem.
No comments:
Post a Comment